DNA Translation and Protein Translocation
mRNA transport from the nucleus
After the mRNA is processed, it must be transported out of the nucleus to the cytoplasm where translation will take place. In order for this to happen, the mRNA associates with a variety of proteins to form a messenger ribonucleoprotein (mRNP). It is in this form that the mRNA exists the nucleus. It exits through nuclear pore complexes in the nuclear envelope. An exosome degrades any improperly processed RNA left behind so that only properly formed mRNA gets exported.
Codons
Three-nucleotide long sequences called codons on mRNAs code for amino acids. There are 64 codons. Sixty-one of them code for amino acids including AUG which is a start codon corresponding to the amino acid methionine. Three codons are referred to as STOP codons. Stop codons terminate translation of mRNA.
The three stop codons are UGA, UAA, and UAG. Remember,
UGA - "You go away"
UAA - "You are away"
UAG - "You are gone"
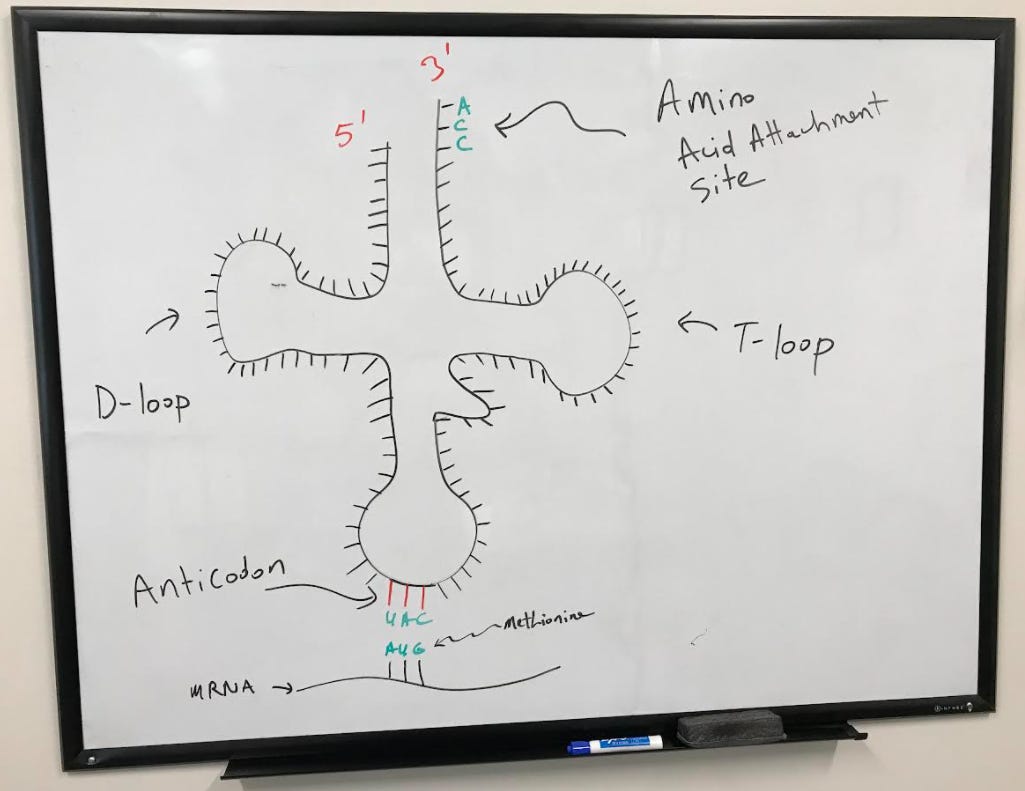
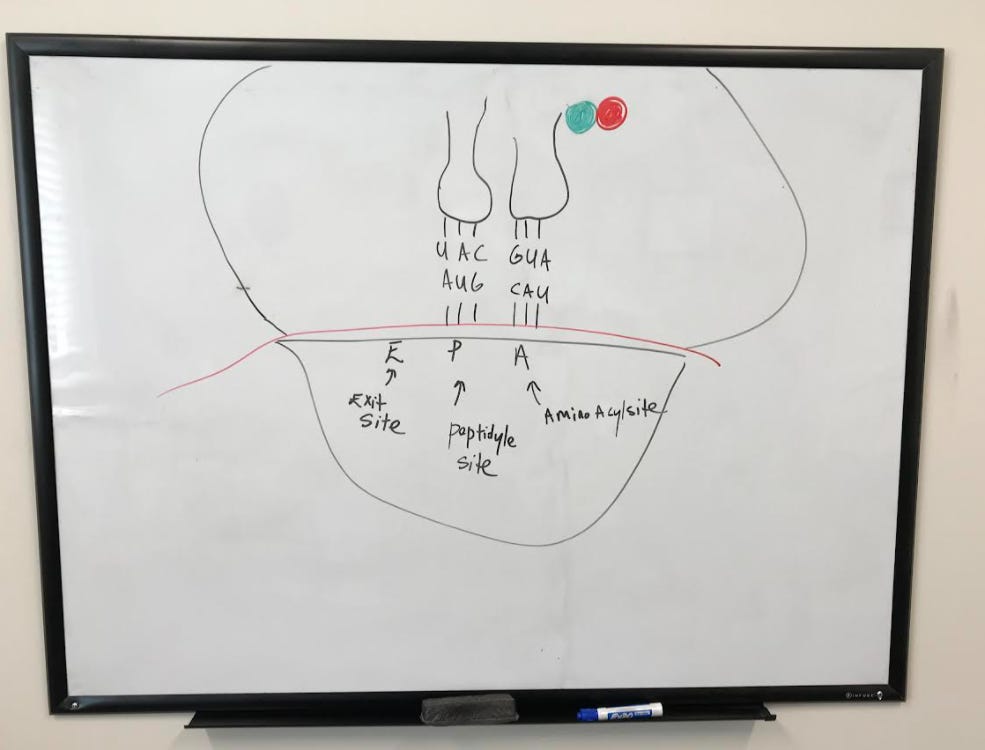
Structure of the tRNA
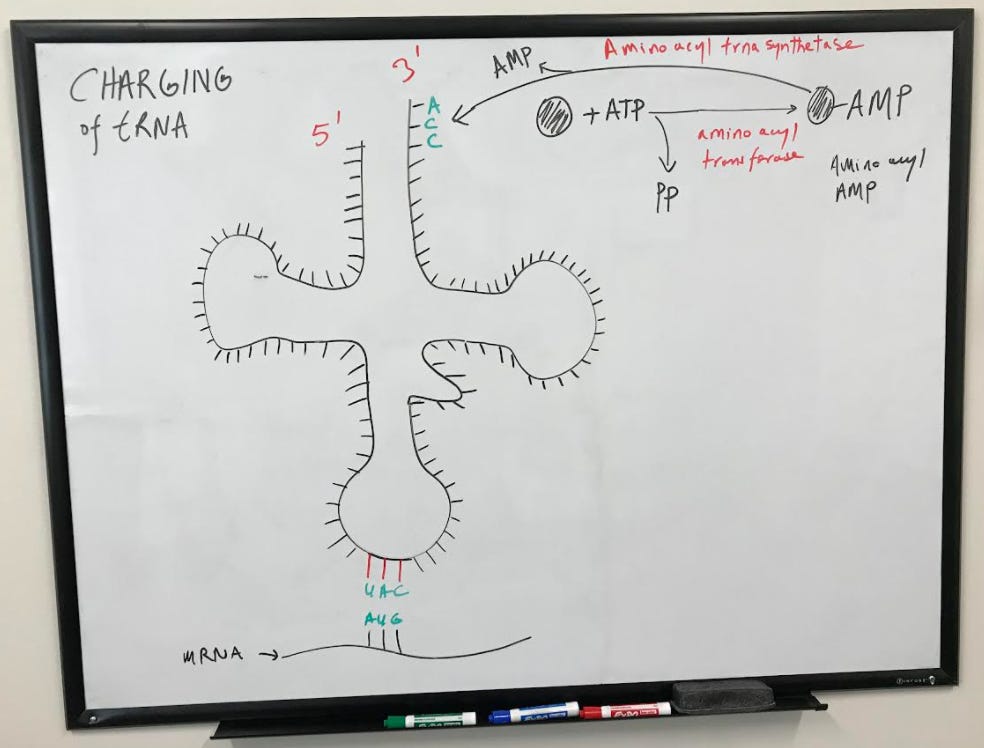
tRNA (transfer RNA) molecules are used during translation to transfer amino acids corresponding to specific codons on the mRNA. As you can see from the figure below, the tRNA forms the shape somewhat of a "t". Amino acids attach to the tRNA at the 3' end where there is the nucleotide sequence, AAC. Two arms called the D and T-loop on the tRNA play an important role in the function of the tRNA and its interaction with the ribosome. The sequence of the tRNA that base pairs specifically with the codon on an mRNA is called the anticodon.
In order to bind the tRNA, amino acids first react with ATP to produce amino acyl-AMP with the help of amino acyltransferase. The amino acid is then transferred to tRNA with the help of aminoacyl tRNA synthetase. AMP is released in the process.
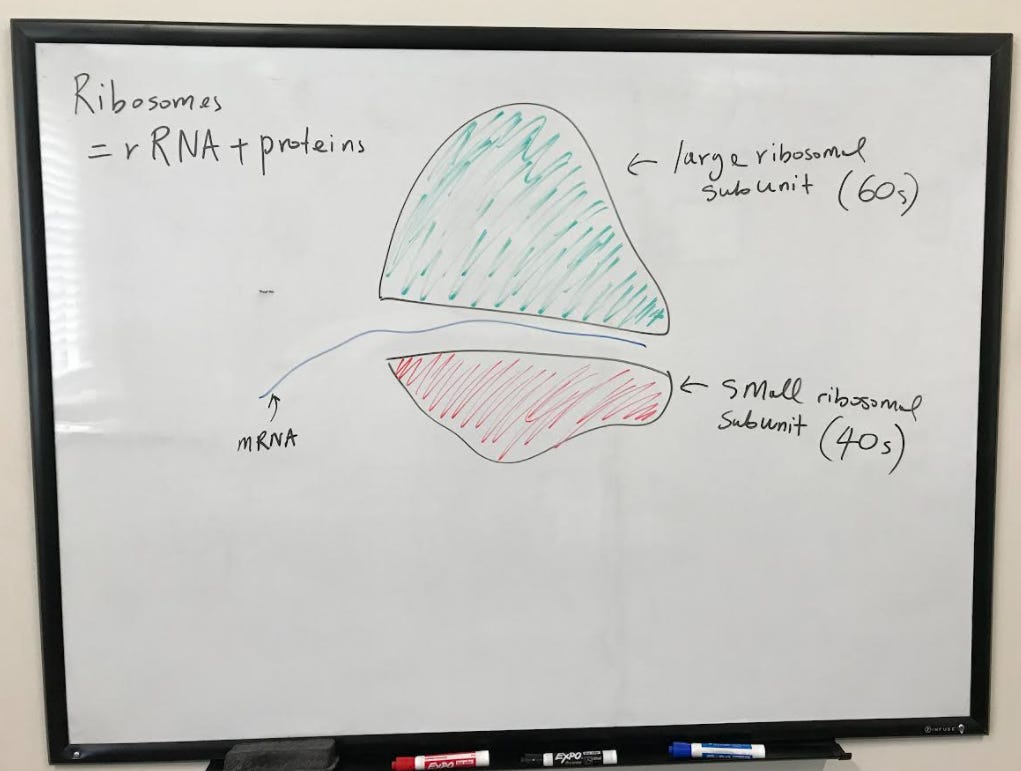
Structure of the Ribosome
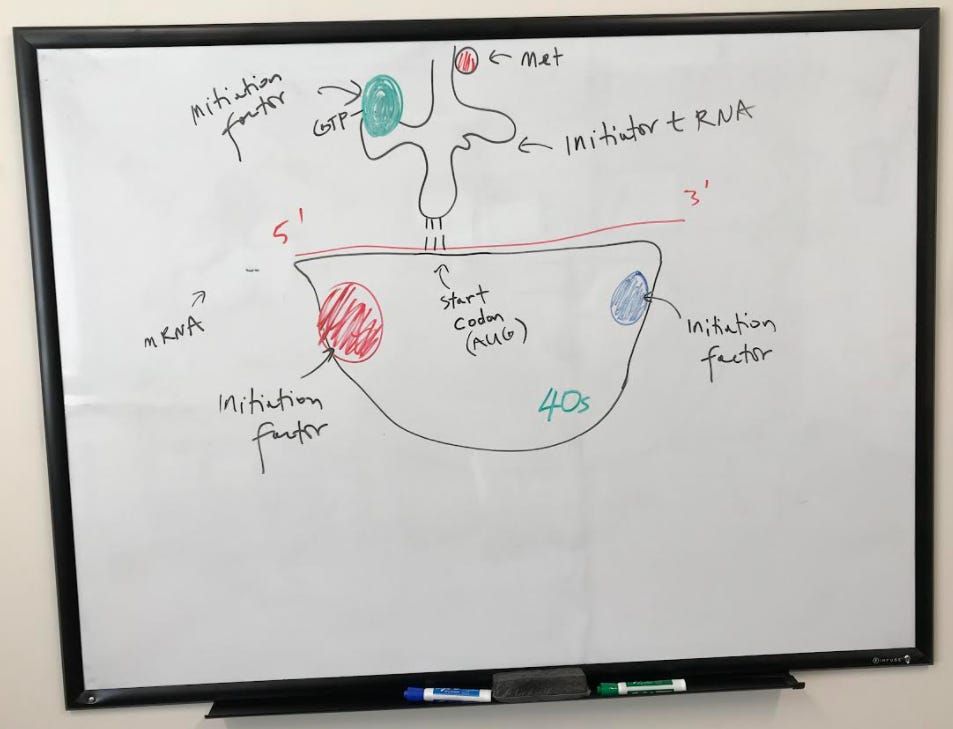
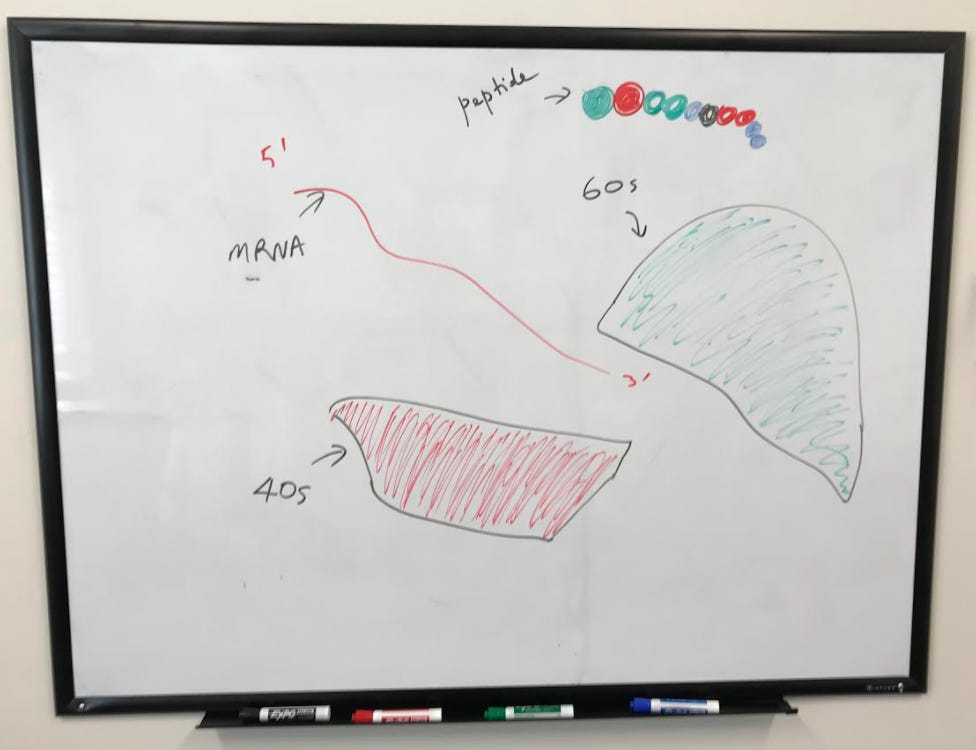
Along with mRNA and tRNA, ribosomes complete the machinery needed for translation. Ribosomes are a combination of rRNA and proteins. There are two subunits of the ribosome, a large and a small. In eukaryotes, the large unit is called the 60S subunit, and the small is called the 40S subunit (“S” is an abbreviation of “Svedberg” – a measure of how the size and shape of a macromolecule moves through a gradient). Prokaryote ribosomes consist of a 50S large subunit and a 30S small subunit.
Steps in Translation
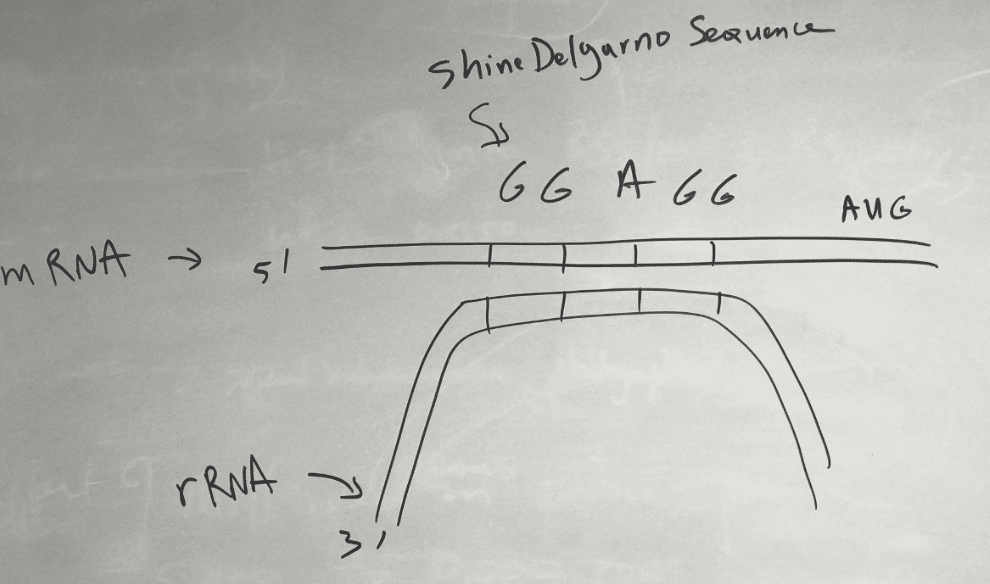
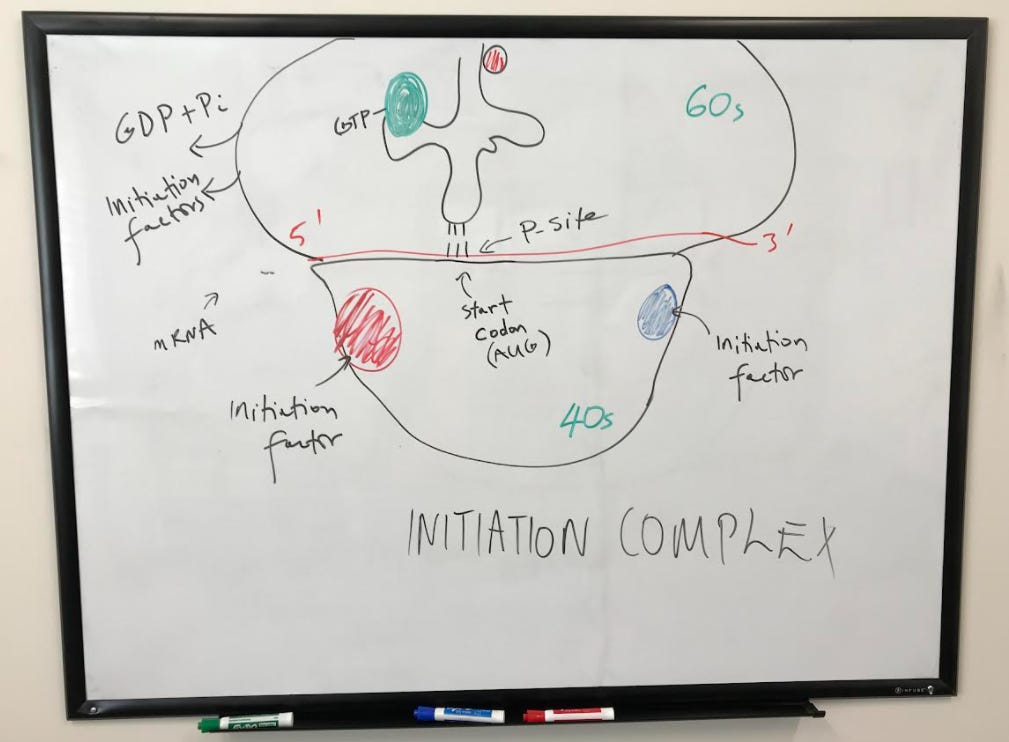
The small subunit positions itself onto the mRNA at the Shine-Delgarno Sequence with the help of initiation factors.
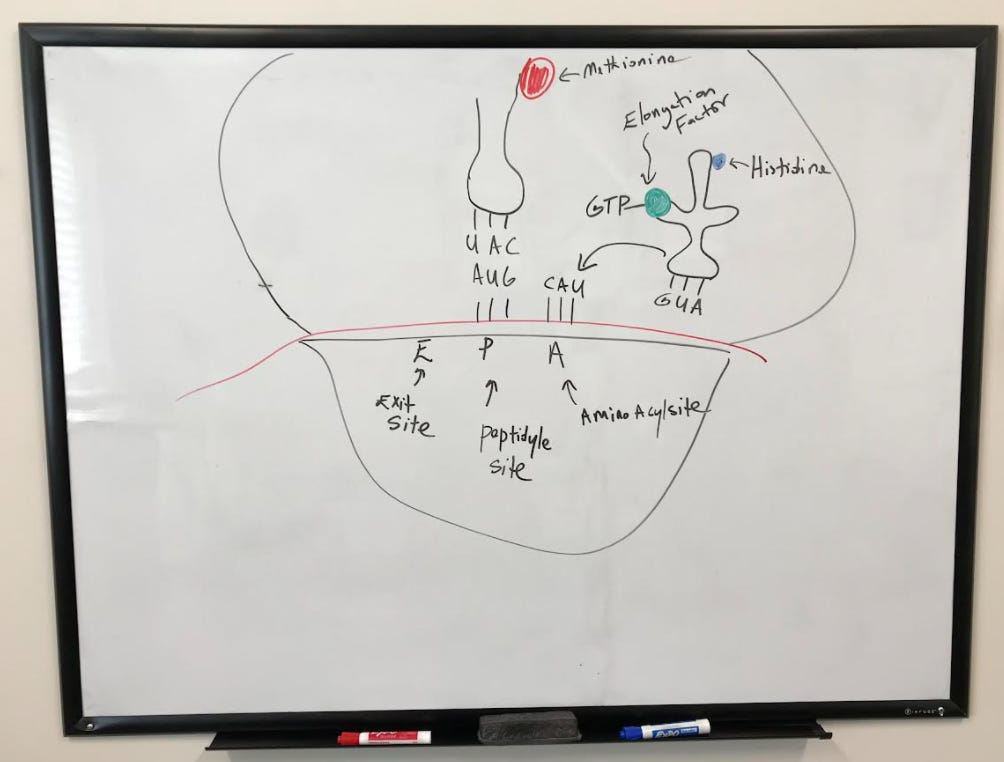
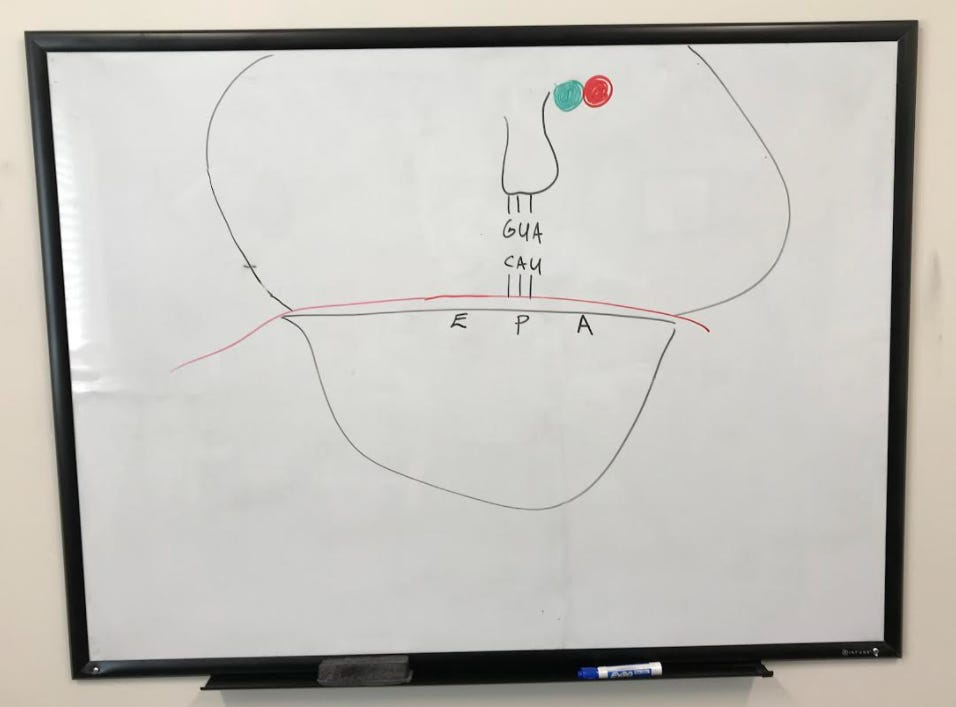
The large subunit is then engaged with the help of initiation factors to complete the initiation complex. A tRNA is now ready to begin translating, starting at the peptidyl site (or p-site) where the start codon AUG codes for methionine.
After the initial installation of the first amino acid (methionine), another tRNA reads the codon on the aminoacyl site (or A site). The movement of tRNA to this site is facilitated by elongation factors.
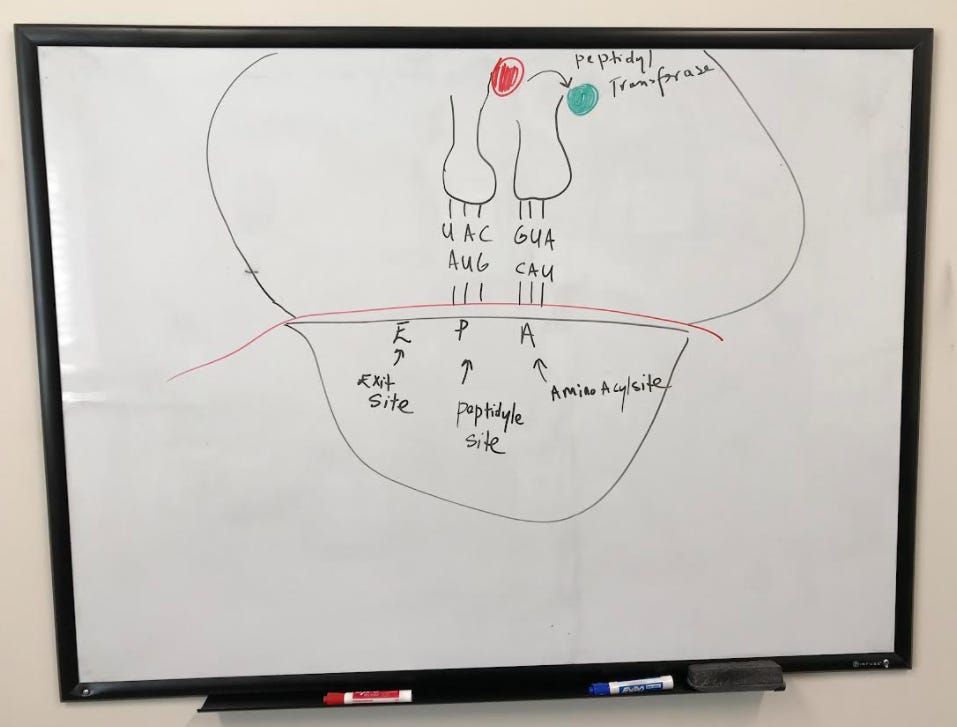
The first amino acid that was translated is transferred to the second using peptidyl transferase.
Now tRNA in the p-site is empty (has no amino acid) while the second has two amino acids. This is the beginning of a chain of amino acids that will make the new protein.
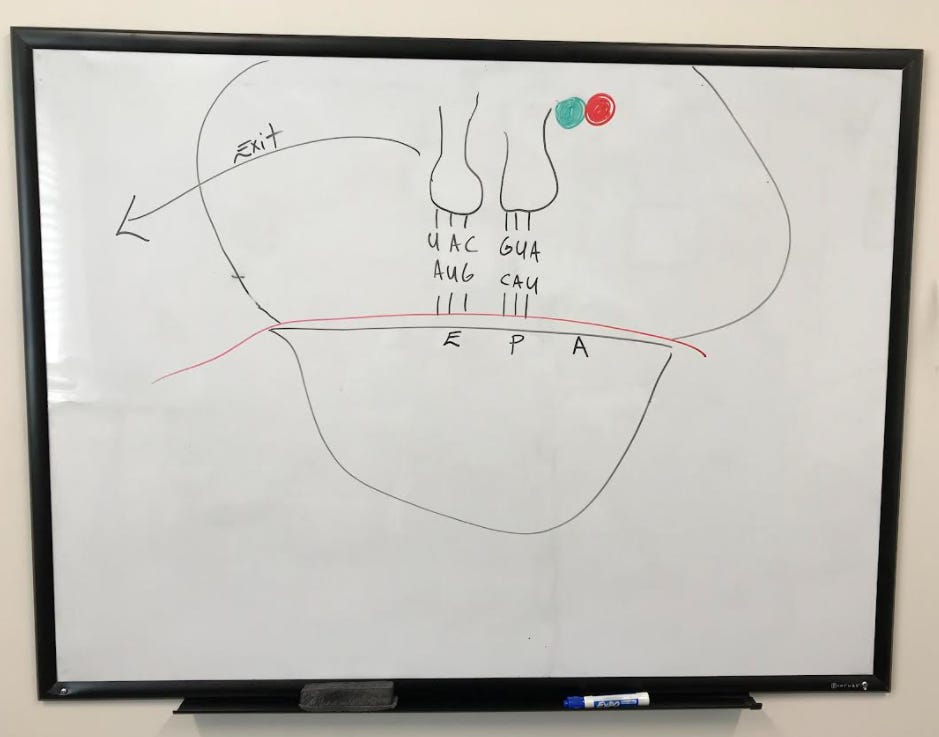
The "empty" tRNA moves over to the exit (or e-site) while the tRNA that was previously in the a-site moves over to the p-site.
The empty tRNA in the e-site then exits the complex, leaving behind the tRNA in the p-site.
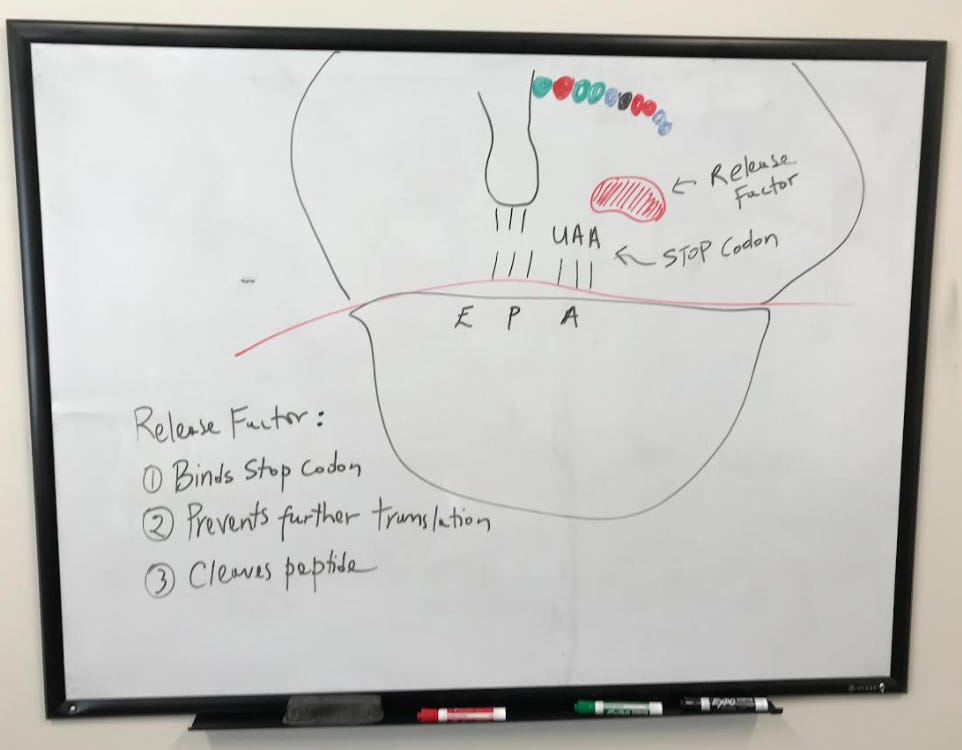
The process continues with a string of amino acids being joined together each time a new tRNA enters the A site. Eventually, a STOP codon is reached. No tRNA binds to these codons.
Instead, a release factor comes in and binds the STOP codon resulting in the release of the protein and a dissociation of the complex.
Protein Translocation
Proteins translated in the cytoplasm either remain there or are translocated to the mitochondria, nucleus, cell membrane, endoplasmic reticulum (ER), endosomes, lysosomes and destinations outside of the cell. Most proteins with final destinations other than the cytoplasm are translated by membrane bound ribosomes in the ER, then packaged into vesicles that migrate and fuse with the Golgi apparatus. In the Golgi, the proteins are modified (e.g. glycosylated) and packaged into vesicles that are shipped to various locations in the cell.
How proteins get into the ER
There are two modes of transport for proteins getting into the ER. There is post-translational translocation and co-translational translocation. In post translational translocation, proteins are translated by free ribosomes in the cytoplasm and are bound by chaperones that prevent them from folding. A hydrophobic region on the protein then binds to the Sec61 translocon protein on the ER membrane and the protein is pulled into the ER by a chaperone protein called binding immunoglobin protein (BIP).
In co-translational translocation, proteins are channeled into the lumen of the ER while it is being synthesized by membrane-bound ribosomes. The proteins are first directed to the ER by a signal sequence of hydrophobic amino acids. A signal recognition particle (SRP) binds the protein as it emerges from the ribosomes and guides it through a channel in the surface of the ER with the help of an SRP receptor.